Our Commitment to Gastrointestinal Health
At Geneoscopy, we are committed to advancing the science of diagnostics for gastrointestinal health.
70Million
Americans¹
250,000
Deaths Annually¹
$140Billion
Medical Costs¹
Approximately 70 million Americans are affected annually by gastrointestinal diseases. Within the oncology space, colorectal cancer alone results in 250,000 deaths annually and $140 billion in medical costs.¹ Additional disease that have high morbidity and mortality within the gastrointestinal space include inflammatory bowel disease, celiac disease, among others. Since the foundation of Geneoscopy, our goal has been to bridge the gap between patients and providers to improve health outcomes for individuals suffering from gastrointestinal disease.
To effectively manage gastrointestinal health, it is important to evaluate the cells lining the gastrointestinal tract, called enterocytes. There are two methods to isolate these enterocytes:
- Endoscopic procedure (i.e., colonoscopy, upper endoscopy, or sigmoidoscopy) to obtain a biopsy.
- Isolation of the enterocytes from stool samples.
Endoscopic procedures are inconvenient, invasive, and expensive, so many patients avoid them. For example, in colorectal cancer, up to 40% of eligible patients fail to get screened via colonoscopy, primarily due to the inconvenience and invasiveness of the procedure.² Our unique stool-based noninvasive solution can reduce compliance barriers for all patients.
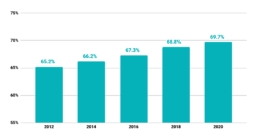
Percentage of U.S. Adults Age 50-75 years Up-to-Date with CRC Screening, Behavioral Risk Factor Surveillance System
Centers for Disease Control and Prevention (CDC). Behavioral Risk Factor Surveillance System Survey Data. Atlanta, Georgia: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, [2012, 2014, 2016, 2018, 2020].
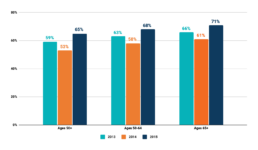
CRC Screening Among Adults Aged 50-75 Years, US, 2013-2018, National Health Interview Survey
Siegel RL, Miller KD, Sauer AG, et al. Colorectal cancer statistics, 2020. CA: A Cancer Journal for Clinicians. https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21601
