Closing the Gap: Why Healthcare Needs More Gender Diversity in Leadership

"Embrace your flaws: Everyone has flaws. I feel that I have been successful because I am able to recognize these flaws, understand where I need help, and fill in the gaps with resources and other people who are better than me in those areas. If you always assume you know everything or are always making the right decision you will be opening yourself up to failure in so many ways."
Dr. Erica Barnell, Chief Science Officer and co-founder of Geneoscopy Tweet
Original interview - published in Health Care IT Today | July 26, 2023
Women comprise 70% of the healthcare workforce and 59% of medical, biomedical, and health sciences graduates, yet are the minority at leadership levels — holding only 25% of senior executive roles. As a result, the lack of women in significant decision-making positions is evident.
According to U.S. Census estimates, no single ethnic or racial group will represent a majority of the U.S. population by 2055. The potential for more positive patient care experiences, greater innovation, and improved organizational performance exists by creating more balance in healthcare leadership.
Existing Disparities and Challenges
Over a century ago, women founded, led, and operated hospitals. Today, they comprise most of the healthcare workforce — but not in leadership positions.
Women represent only 25% of top leadership roles in healthcare while holding an even lower percentage of corporate board seats (~14%) for publicly held life sciences companies. Within privately held companies, these numbers are even more underwhelming.
Despite significant advancements, we have a long way to go to address existing gender disparities in education and careers within STEM. As few as ten years ago, I was one of only a handful of women scientists in research labs and often the only woman in the boardroom. During presentations, I was asked more questions about my personal life than my business ideas — questions I have never heard asked of my male peers. Additionally, when defending research results, questions were often directed to my male peers even though I was the subject matter expert.
Gender Bias in Medical Research
Women’s health is often considered a niche area. But how can half the world’s population be considered niche? Of the nearly $42 billion the National Institute of Health (NIH) funds annually on health research, only $5 billion is spent studying women’s health issues in research labs led by men.
These numbers are consistent with female representation in clinical trials. On average, 41.2% of study participants are female. This number is often lower than the number of females typically diagnosed with the disease being studied. Unconscious biases can also significantly impact gender and racial equity in research studies. For example, a recent study showed that 80% of black women with breast cancer expressed willingness to participate in clinical trials; however, only 40% were included.
In colorectal cancer research, clinical studies have traditionally been held at large academic or endoscopy centers where patients typically have a colonoscopy scheduled, are health literate, and have insurance. This sample does not accurately reflect the population most at risk for developing colorectal cancer due to a lack of screening.
Researchers must adopt alternatives to traditional research methods to impact health equity positively. One approach is using decentralized recruitment efforts for clinical trials to support engaging diverse populations at greater risk for disease. For example, using social media to recruit study participants can help reach underserved communities and requires a low marketing dollar commitment. Decentralizing clinical trials can improve prevention and treatment for all populations, particularly racially and ethnically diverse communities.
The Importance of Women Leaders in Healthcare
Decades of research have shown that when women leaders are empowered to lead, everyone benefits. Fiscally, studies indicate that companies with women in top leadership positions are valued at $42 million higher than other companies. They generate a 10.1% return on equity annually versus 7.4% for those without female leadership.
When looking beyond the bottom line, including women in a leadership team increases a company’s global human capital wealth by 22%, with substantial health, social, and economic gains. The presence of women leaders helps achieve better results, including increased productivity, improved collaboration, greater commitment, and a fair work experience. Women are considered encouraging in a collaborative environment and are more honest, compassionate, outgoing, and creative.
What Does the Future Hold?
We are already seeing glimpses into the future with advances in digital technologies, including artificial intelligence, virtual and augmented reality, 3D printing, robotics, gene sequencing, and nanotechnology. As this unfolds, healthcare leaders must hire, train, and lead increasingly diverse teams of healthcare professionals along with teams from product development, training, tech, engineering, and manufacturing/construction to ensure inclusivity. But most importantly, leadership must align all decisions and directions to ensure the patient always comes first.
Witt/Kieffer discovered that 84% of survey respondents agreed that one of the best ways to support these endeavors is to establish mentorship programs that track to leadership roles. They offered these tips to create a successful mentorship program:
- Provide role models that help with advancement
- Develop a robust mentorship program
- Create internship opportunities
Advice For Future Women Healthcare Leaders
Trying to live up to someone else’s ideals will only cause you to sacrifice your own and block your path to the top. To be the best version of yourself, which includes being a fantastic leader, set your expectations and live up to those.
Here are three pieces of advice to guide you both personally and professionally:
- Be resilient and have humility: Adversity is a given. Keeping a positive attitude, being optimistic, controlling emotions, and seeing obstacles and failures as opportunities are keys to learning, improving, and growing.
- Look for opportunities, take risks, push yourself, and go for it: Successful leaders are proactive. Don’t wait for others to reach out to you – build and develop the relationships you need to make your goals happen.
- Success means finding YOUR balance: Life and work are often not in harmony in the real world. Living a successful and balanced life is more about knowing what motivates you and having tough conversations with people who matter most in your life.
While things are improving, there’s work ahead to close the gender gap in healthcare leadership. Prioritizing an inclusive and safe environment where people acknowledge the experience and expertise of women at all levels is essential to creating the culture shift necessary to make change happen throughout our industry.
 About Erica Barnell
About Erica Barnell
Erica Barnell graduated from Cornell University with a dual degree in Biological Sciences and Applied Economics & Management. Dr. Barnell completed her MD PhD at the Washington University School of Medicine with a PhD focus in Molecular Genetics and Genomics. Her thesis work surrounded the development of bioinformatics tools to alleviate the analysis bottleneck within precision oncology. Dr. Barnell has published 24 peer-reviewed manuscripts, four patents, and two book chapters within the oncology space. Dr. Barnell conducted the original research to validate Geneoscopy’s stool extraction method and managed all clinical studies for Geneoscopy’s colorectal cancer screening test. Dr. Barnell has contributed to the existing engagement with the FDA, which includes submitting a pre-market approval application, holding three presubmission meetings, completing BIMO/PAI audits, and obtaining Breakthrough Device Designation for the lead colorectal cancer (CRC) screening test. Dr. Barnell was named to the Forbes 30 Under 30 list in 2020 for her contribution to the Healthcare space.
Geneoscopy receives Z-Code from Palmetto GBA for its Noninvasive Multi-Target Stool RNA Colorectal Cancer Screening Test
ST. LOUIS, April 12, 2023 / Geneoscopy, Inc., a life sciences company focused on the development of diagnostic tests for gastrointestinal health, today announced the assignment of a unique DEX Z-code from Palmetto GBA’s MolDX program for the company’s noninvasive multi-target stool RNA (mt-sRNA) colorectal cancer screening test.
“Obtaining this Z-code is vital for market access and aligns with our plans for commercial launch,” said Andrew Barnell, Chief Executive Officer of Geneoscopy. “Once approved and launched, we anticipate Geneoscopy’s noninvasive mt-sRNA colorectal cancer screening test will offer patients and healthcare providers a convenient and reliable CRC screening option for earlier detection and treatment.”
The assignment of the DEX Z-code as a test identifier is a significant step as Geneoscopy awaits the US Food and Drug Administration’s decision regarding the PMA submitted in January 2023. Geneoscopy’s test is not FDA approved and not available for sale.
About Geneoscopy Inc.
Geneoscopy Inc. is a life sciences company focused on developing diagnostic tests for gastrointestinal health. Leveraging its proprietary, patented stool-derived eukaryotic RNA (seRNA) biomarker platform, Geneoscopy’s mission is to empower patients and healthcare providers with the confidence to detect, prevent and monitor disease with our innovative tests developed to transform gastrointestinal health. Beyond colorectal cancer screening, Geneoscopy is developing tests for diagnosis, treatment selection, and therapy monitoring in other disease areas in partnership with leading universities and biopharmaceutical companies. For more information, visit www.geneoscopy.com and follow the company on LinkedIn.
Geneoscopy Inc. Forward-Looking Statements
This release includes information about Geneoscopy’s future plans concerning its noninvasive molecular test that can detect colorectal cancer and precancerous adenomas, which constitute forward-looking statements. These forward-looking statements are based on the Company’s reasonable estimates of future results or trends. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict, many of which are outside the Company’s control. Geneoscopy’s actual results and financial condition may differ materially from those in the forward-looking statements. Although the Company believes its business plans and objectives reflected in or suggested by these forward-looking statements are reasonable, such plans or objectives may not be achieved. The actual results may differ substantially from the projected result.
MEDIA CONTACT
Judy Pretto
Director, Marketing Communications
815.534.0521
Oncology Tube Interview with Dr. David Lieberman, OHSU
Dr. David Lieberman discusses the Geneoscopy: Efficacy of Non-Invasive Colorectal Cancer Screening Test Evaluated in the CRC-PREVENT Phase 3 Trial
Geneoscopy Joins White House for Cancer Moonshot Colorectal Cancer Forum

“As leaders, we have a responsibility to come together to impact cancer. Colorectal cancer is one of the most preventable and treatable cancers when diagnosed early. Geneoscopy is honored to contribute to the efforts to address disparities and increase access to screening alternatives by developing highly effective noninvasive screening tests and personalized patient journeys that can save more lives.”
Andrew Barnell, Geneoscopy CEO and co-founder
March 10, 2023 (St. Louis, MO) | Geneoscopy Chief Executive Officer and co-founder Andrew Barnell is joining colorectal cancer (CRC) advocacy and industry leaders from across the country to attend the Cancer Moonshot Colorectal Cancer Forum, hosted by the White House on March 10. Together a national coalition of insurers, industry, federal agencies, health care providers, retail businesses, and CRC patients, will highlight existing successes in CRC screening and prevention access, identify opportunities to improve CRC screening for all communities and discuss advances in targeted treatment options.
In February 2022, President Biden reignited the Cancer Moonshot with renewed leadership and ambitious goals to “end cancer as we know it.” To address colorectal cancer and assist with increasing screening efforts, Mr. Barnell has joined national advocacy organization Fight Colorectal Cancer (Fight CRC) along with leading patient advocacy organizations and industry leaders in the CRC community.
In addition to the forum, Mr. Barnell will join Fight CRC’s coalition dedicated to developing a report that will outline a blueprint to address the serious equity gaps facing communities and affecting colorectal cancer prevention in America, highlighting the patient screening journey and setting bold metrics to increase colorectal cancer screening and improve patient care.
“As leaders, we have a responsibility to come together to impact cancer.” said Mr. Barnell. “Colorectal cancer is one of the most preventable and treatable cancers when diagnosed early. Geneoscopy is honored to contribute to the efforts to address disparities and increase access to screening alternatives by developing highly effective noninvasive screening tests and personalized patient journeys that can save more lives.”
Colorectal cancer is currently the second leading cause of cancer-related deaths in the U.S. For people younger than 55, colorectal cancer diagnosis has doubled from 11% (1 in 10) in 1995 to 20% (1 in 5) in 2019, according to a recent report published by the American Cancer Society CA: Cancer Journal for Clinicians.
“Our focus at the Cancer Moonshot Colorectal Cancer Forum is to advocate for taking action, not just talk about it but operationalize change,” said Anjee Davis, Fight CRC President. “We need to dig deeper into the systemic and behavioral patterns that accompany colorectal cancer from screening to treatment and identify the best opportunities to intervene, educate and – ultimately – drive large-scale advancements. We have the momentum to do this, and it will ultimately save lives.”
The forum will be closed to media; however, it will be live-streamed by the White House at wh.gov/live, and for photos, b-roll, or official comments before or after the forum, please contact media@fightcrc.org or media@geneoscopy.com.
About Fight Colorectal Cancer
Fight Colorectal Cancer (Fight CRC) is a leading patient-empowerment and advocacy organization in the United States, providing balanced and objective information on colon and rectal cancer research, treatment, and policy. We are relentless champions of hope, focused on funding promising, high-impact research endeavors while equipping advocates to influence legislation and policy for the collective good. Learn more at FightCRC.org.
About Geneoscopy, Inc.
Geneoscopy Inc. is a life sciences company focused on developing diagnostic tests for gastrointestinal health. Leveraging its proprietary, patented stool-derived eukaryotic RNA (seRNA) biomarker platform, Geneoscopy’s mission is to empower patients and providers to transform gastrointestinal health through innovative diagnostics. Beyond colorectal cancer screening, Geneoscopy is developing tests for diagnosis, treatment selection, and therapy monitoring in other disease areas in partnership with leading universities and biopharmaceutical companies. For more information, visit www.geneoscopy.com and follow the company on LinkedIn.
Geneoscopy Inc. Forward-Looking Statements
This release includes information about Geneoscopy’s future plans concerning its noninvasive molecular test that can detect colorectal cancer and precancerous adenomas, which constitute forward-looking statements. These forward-looking statements are based on the Company’s reasonable estimates of future results or trends. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict, many of which are outside the Company’s control. Geneoscopy’s actual results and financial condition may differ materially from those in the forward-looking statements. Although the Company believes its business plans and objectives reflected in or suggested by these forward-looking statements are reasonable, such plans or objectives may not be achieved. The actual results may differ substantially from the projected result.
Media Contact
Judy Pretto
Director, Marketing Communications
815.534.0521
media@geneoscopy.com
Health Tech: Erica Barnell On How Geneoscopy's Technology Can Make An Important Impact On Our Overall Wellness

"Embrace your flaws: Everyone has flaws. I feel that I have been successful because I am able to recognize these flaws, understand where I need help, and fill in the gaps with resources and other people who are better than me in those areas. If you always assume you know everything or are always making the right decision you will be opening yourself up to failure in so many ways."
Dr. Erica Barnell, Chief Science Officer and co-founder of Geneoscopy
Tweet
Original interview with Yitzi Weiner - published in Authority Magazine | July 11, 2022
In recent years, Big Tech has gotten a bad rep. But of course, many tech companies are doing important work making monumental positive changes to society, health, and the environment. To highlight these, we started a new interview series about “Technology Making An Important Positive Social Impact”. We are interviewing leaders of tech companies who are creating or have created a tech product that is helping to make a positive change in people’s lives or the environment. As a part of this series, I had the pleasure of interviewing Erica Barnell, PhD.
Geneoscopy co-founder, Erica Barnell graduated from Cornell University with a dual degree in Biological Sciences and Applied Economics & Management. Erica is an MD/PhD candidate at the Washington University School of Medicine. In 2019, Erica completed her PhD at the McDonnell Genome Institute. Her thesis work surrounded the development of bioinformatic tools to alleviate the analysis bottleneck within precision oncology. Erica has published 19 peer-reviewed manuscripts, 11 abstracts with 4 platform presentations, and two book chapters within the oncology space. Erica conducted the original research to validate Geneoscopy’s stool extraction method and managed all feasibility studies for Geneoscopy’s colorectal cancer (CRC) screening test. Erica was responsible for existing engagement with the FDA, which includes holding three presubmission meetings and obtaining Breakthrough Device Designation for the lead CRC screening test. Erica is the lead inventor on all patents pertaining to Geneoscopy’s technology and is currently the site investigator on two IRB-approved protocols.
Thank you so much for joining us in this interview series. Before we dive in, our readers would love to learn a bit more about you. Can you tell us a bit about your childhood backstory and how you grew up?
I was born and raised in St. Louis, Missouri. Both of my parents are lawyers by trade, but my father left his law firm many years ago and started a company called Precision Practice Management that improves healthcare billing for physicians and hospitals. I grew up with my brother (Andrew Barnell) who became my partner in crime at a young age. Together we attended John Burroughs High School (the same one as John Hamm!) and Cornell University. In 2013, I graduated from Cornell with a double major in Biological Sciences and Applied Economics and Management. After college, while I was applying for medical school, I moved to Israel and worked at Tel Aviv University where I was developing a vaccine for Shigella. It was during this time that I fell in love with using science to develop therapeutics and diagnostics that improve the ways that we deliver healthcare. I matriculated to the MD/PhD program at Washington University in St. Louis in 2014. For the research component of my degree, I entered the gastrointestinal space where our team was looking at ways to improve malnutrition for children in Africa. To do this, we were identifying human transcriptome changes in diapers that would indicate disease, providing interventions for children that had a high disease burden, and alleviating the impact of gastritis on nutrient procurement. Simultaneously, for the medical component of my degree, I was learning about different diseases and completing clinical rotations to learn about how doctors treat patients. During this clinical component, I met a woman in her 50's who presented to the hospital with Stage IV colorectal cancer. The woman had never been screened for colorectal cancer because she could not take time off work to have a colonoscopy. Based on the research I conducted in the microbiome labs, I realized that there had to be a better way to help people identify disease before it became deadly. At the time I called my brother, who had just recently received his MBA from Wharton and we co-founded Geneoscopy to start thinking about ways to address this issue.
Can you share the most interesting story that happened to you since you began your career?
I have tried to be very active in the St. Louis entrepreneurial community since we launched Geneoscopy in 2016. As such, I like to volunteer at local conferences whenever possible. One day I was asked to attend a local roundtable event with a few entrepreneurs to discuss the development and needs of the city. I showed up not really knowing exactly what was going to happen, so I showed up a little early and found my place at the small table in the conference room. After a few minutes, the door to the conference room opened up and to my surprise, Mark Zuckerberg walked in. He sat right across from me and said, “Hi I’m Mark”. We proceeded to have the most wonderful afternoon learning about what is going on at Facebook and discussing the entrepreneurial community in St. Louis. It was so unexpected but pivotal point in my career where I realized that all entrepreneurs share some of the same experiences and obstacles and I felt very motivated to continue the important work we are conducting at Geneoscopy to improve the way that we treat GI disease.
None of us can achieve success without some help along the way. Is there a particular person who you are grateful for who helped get you to where you are? Can you share a story about that?
For the MD/PhD program at Washington University, I was tasked with identifying a mentor or principal investigator for my PhD training. I remember spending many hours searching online for a laboratory that I wanted to join that would help me achieve my goals as both a medical/graduate student as well as an entrepreneur. I remember reviewing Obi Griffith’s laboratory and being so inspired by the work he was doing to advance precision oncology using bioinformatic tools and computer science. Despite having never taken a didactic course in coding or computer science, I asked to speak with him about joining his lab. On our first interview, he asked me what language I spoke (as in, what computer language), and I said French; I was obviously not off to a good start. I am not entirely sure what Obi saw in me that made him take a chance on me, but he offered me a position in the lab for my PhD training. Accepting that position was one of the best decisions I have ever made. My education in his laboratory directly contributed to who I am today and to the success of Geneoscopy. Under his guidance, I have published 20 peer-reviewed manuscripts, 14 first-author abstracts with 4 platform presentations, two book chapters in the precision oncology space, and 3 patents that are related to the company. Obi was supportive of both my initiatives as a scientist in his lab as well as my desire to pursue a career as an entrepreneur. I am truly grateful for his mentorship over the last 7 years and hope that we continue to work together to solve very important problems in the precision oncology space.
Can you please give us your favorite “Life Lesson Quote”? Can you share how that was relevant to you in your life?
“If you want to take the island burn the boats” — This is a quote by Julius Caesar where he was describing that if you want to capture an island, you must burn your own boats (or rather your personal safety nets) so that you have no choice other than success. I have found that entrepreneurship is incredibly volatile whereby one day I will feel confident that we are on the right path and the next day I feel that our efforts are futile. As a leader, and especially when you are fighting for a very important cause, it can be necessary to push through difficult times. This quote reminds me that achieving your goals is not easy and that making change in this world takes unwavering passion and persistence.
You are a successful business leader. Which three-character traits do you think were most instrumental to your success? Can you please share a story or example for each?
Embrace your flaws: Everyone has flaws. I feel that I have been successful because I am able to recognize these flaws, understand where I need help, and fill in the gaps with resources and other people who are better than me in those areas. If you always assume you know everything or are always making the right decision you will be opening yourself up to failure in so many ways.
Never procrastinate: Each morning, I wake up and make a list of activities for the day and then attack the most difficult thing on the list. Getting things done early and finishing the difficult things keeps me motivated so that I never feel like I am falling behind.
Perfection is the enemy of done: At the company, I have learned that there is a “good enough rule” whereby once you reach a certain level of acceptance, you must let go and move on. Whether it is a grant or a manuscript or even an email, I see a lot of people attempting to achieve perfection and will go over the same materials hundreds of times only to make marginal changes that do not dramatically add value to the work. For me, it is more important to make sure that everything gets done even if it is not 100% perfect.
Ok super. Let’s now shift to the main part of our discussion about the tech tools that you are helping to create that can make a positive impact on our wellness. To begin, which problems are you aiming to solve?
Geneoscopy is developing next-generation diagnostics that leverage the power of stool-derived eukaryotic RNA (seRNA) to better prevent, detect, and guide treatment for gastrointestinal disease. Our lead product is a noninvasive diagnostic to detect colorectal cancer and precancerous adenomas in average-risk patients, those individuals over the age of 45 years old.
How do you think your technology can address this?
Geneoscopy has developed a novel method to extract eukaryotic RNA biomarkers from stool samples to enable a new wave of noninvasive diagnostic tests to prevent, detect, and treat gastrointestinal (GI) disease. While RNA has been shown to be a very powerful biomarker in the GI space, previous attempts to preserve and isolate human RNA in stool have been unsuccessful due to the unstable nature of these biomarkers. The company has created a proprietary method to preserve RNA in stool at ambient temperature that allows patients to assess their GI health in the comfort of their own homes.
Can you tell us the backstory about what inspired you to originally feel passionate about this cause?
When I started medical school in 2014, my research at the time was mostly dedicated to solving micronutrient malnutrition for children in Africa. We received a large Bill and Melinda Gates Foundation Grant to understand a disease called Environmental Enteropathy (EE), which afflicted children under the age of 5 and caused growth faltering even in the presence of adequate nutrition. For these children, we were not able to provide colonoscopies to obtain biopsies, so instead, I was tasked with developing a noninvasive method to identify which children had the disease and which children were healthy. To do this, we collected dirty diapers and isolated the eukaryotic, or human cells to identify the disease. Once we were able to successfully identify which children were afflicted with EE, we could provide medication and alleviate the malnutrition. This effort was largely successful and was part of Bill Gates’s keynote speech at the 2018 JP Morgan Healthcare Conference! Simultaneously, while I was completing this research at the Washington University School of Medicine, I had been executing clinical rotations in Barnes-Jewish Hospital. During this time, I met a 52-year-old woman who presented to the hospital with Stage IV colorectal cancer. When I asked the woman why she had never received a colonoscopy, she stated that she could not take time off work to have the procedure done. It was distressing to me that we live in a world where we can sequence the genome in 24 hours, can provide life-saving therapeutics to solve many different types of cancers, but we couldn’t provide this woman with an alternative to a colonoscopy. I realized that the research I had been doing in the microbiome space could potentially be the solution to this compliance problem in the CRC space. A few months later I founded Geneoscopy with the goal to improve the way that we look at gastrointestinal disease.
How do you think this might change the world?
Colorectal cancer is the second leading cause of cancer-related deaths in this country with over 50,000 deaths each year. This paradigm is mostly related to low compliance for colonoscopy and subsequent late-stage diagnosis of disease. Colorectal cancer is unique in that it typically starts as a small polyp that can grow over the course of several years. If the precancerous polyp is detected before malignant transformation, the patient has almost a 100% 5-year survival. However, if the polyp goes undetected, then it can become cancerous with a massive increase in morbidity and mortality.
While there are some noninvasive tests on the market that attempt to address compliance with colonoscopy, most of these tests are aimed at detecting cancer, rather than preventing cancer through polyp detection. The high false negative rate for polyps, especially for advanced adenomas, has major implications for patient care. Geneoscopy has developed a diagnostic that specifically addresses this concern. Improved sensitivity for advanced adenomas with maintenance of sensitivity for colorectal cancer provides us with the opportunity to lower morbidity and mortality associated with CRC through improved CRC screening compliance and subsequent reduction in the number of late-stage diagnoses.
Keeping “Black Mirror” and the “Law of Unintended Consequences” in mind, can you see any potential drawbacks of this technology that people should think more deeply about?
All diagnostic tests have false positive and false negative rates. A false positive test is when you have a positive test on the diagnostic, but there are no actual findings (polyps or cancer) in your body. A false negative test is when you have a negative test on the diagnostic, but your body has a polyp or cancer in the colon. Given that all patients with a positive Geneoscopy test will go on to receive a colonoscopy, a false negative is much more serious. I think it is important for people to listen to their bodies and make sure that even if your doctor or the tests are saying that everything is ok, but you do not feel right then you must be an advocate for your health and make sure that your voice is heard.
Here is the main question for our discussion. Based on your experience and success, can you please share “Five things you need to know to successfully create technology that can make a positive social impact”? (Please share a story or an example, for each.)
“Never pay for something you can get for free” — When you look at Geneoscopy now, we have over 60 employees, an 11,000-square foot laboratory, 3 patents, and over $105M in venture-backed funding. However, in the beginning, we were just three students in a conference room with some grant funding. In those early days, we thought very carefully about how we would plan our budget to ensure that we were adding value to the company. To that end, we always made sure that if there was something we could get for free or for a reduced cost, we took advantage of that opportunity. For example, we would ask distributing companies if we could use a demo or a used machine, we would ask incubators if we could borrow a workbench and pipettes to execute preliminary research. We applied for non-dilutive grants and completed pitch competitions to try and extend our runway. In the end, because we were scrappy, we were able to complete our clinical trials and demonstrate that the technology worked for very little capital. This allowed us to raise further funds to build our lab and execute our pivotal trial for the CRC screening assay.
“Surround yourself with people who think differently than yourself” — There are many leaders who surround themselves with people who are afraid to challenge the status quo or speak up if they see something. I encourage my team to tell me if they think something is going wrong or if I am personally doing something that could be optimized because it prevents us from falling into avoidable traps or pitfalls. At Geneoscopy we have a culture of openness where even the youngest or newest employees hopefully feel welcome to speak up and discuss what they think is best so that we can grow as a company in the right direction.
“If you are going to fail, fail fast” — Especially in science, it is important to set acceptance criteria and stick to them. If you design an experiment, you must think about what it would take to consider the experiment successful and if you do not meet your goals then you move on to the next one. A lot of entrepreneurs become so invested in their ideas that they are afraid to pivot or let go of something that is not working and you can end up investing valuable resources in a dead end.
“Age is just a number” — There are so many times that people have told me that I am ‘too young’ to run a company. It is important to know your limitations and fill in gaps with consultants, advisors, or employees who have been there and done it before, but just because you are young does not mean that you cannot achieve great things.
“Challenge the status quo” — When we launched Geneoscopy, one of the biggest pushbacks was that the gastroenterology community would never accept a non-invasive diagnostic test for CRC screening because it would take away the number of colonoscopies performed per year. We viewed it a little differently; if we could create a test that had incredibly high sensitivity for advanced adenomas, which represented a large portion of the population, then we would be able to funnel high-risk patients to GI offices and allow them to complete complicated cases that provide more revenue per case and more satisfaction to the doctors. When we pitched this idea to the GI community, it became clear that our solution could solve the screening compliance problem while simultaneously allowing GI doctors to continue to run their businesses.
If you could tell other young people one thing about why they should consider making a positive impact on our environment or society, like you, what would you tell them?
For many people, their job is a means to an end. They go to work so they can pay for their hobbies or activities outside of work. I feel very fortunate that I truly enjoy going to work every day. People always say that if you love what you do then you never work a day in your life, and I certainly think that is a true statement. Each day I genuinely feel like I am making a difference and joining in the fight against cancer.
Is there a person in the world, or in the US with whom you would like to have a private breakfast or lunch, and why? He or she might just see this, especially if we tag them. :-)
George Lucas (screenwriter, producer, director, and creator of Star Wars) — Oh my gosh, if I could meet George Lucas I would die! Not only am I personally a huge fan of Star Wars, but he created a vision of healthcare in his Star Wars universe that inspired and shaped how technology has developed here on Earth. The healthcare technology portrayed in George Luca’s Star Wars universe can be seen in today’s hospitals and healthcare settings including things like prostheses inspired by Luke Skywalker’s robotic arm. The idea of droid medics is in use in emergency rooms right now for stroke patients. It’s called Telestroke and it allows doctors to treat stroke patients from miles away, and the concept of Force healing (using the power of the mind as a healing tool) is being used for chronic pain management, insomnia, and to manage side effects of cancer treatments.
I would love to know where Mr. Lucas sees science fiction taking healthcare next…like Bacta tanks — where you can be submerged in a fluid that could accelerate healing and treat major injuries. I would also love to know more about his life, too. He has an entrepreneurial spirit — something we both have in common. He dealt with setbacks and challenges along his career path, and learned not to take “no” for an answer.
I think lunch with George Lucas would be nothing less than fascinating.
How can our readers further follow your work online?
Our research can be found on our website at www.geneoscopy.com. We are also very active on social media to promote GI health on the following platforms:
Twitter: @GeneoscopyCo
LinkedIn: https://www.linkedin.com/company/geneoscopy
Facebook: https://www.facebook.com/GeneoscopyCo
Instagram: https://www.instagram.com/geneoscopy/
Thank you so much for joining us. This was very inspirational, and we wish you continued success in your important work.
UPDATE SINCE PUBLICATION:
Since July 2022, Dr. Barnell and the Geneoscopy team have completed the CRC-PREVENT trial to evaluate the efficacy of its noninvasive, stool-based, at-home diagnostic screening test to detect colorectal cancer (CRC) and advanced adenomas (AA) in average-risk individuals. Geneoscopy’s test demonstrated 94% sensitivity for CRC and 45% sensitivity for AA, representing the highest sensitivity profile reported to date for any noninvasive CRC screening test in a prospective clinical study.
“We are committed to closing this gap for patients who require screening and look forward to working with the FDA to bring a convenient and reliable screening option to patients that may allow for earlier detection and treatment – and potentially save lives,” said Dr. Barnell.
In January 2023, based on the trial results, Geneoscopy submitted a Premarket Approval (PMA) Application to the FDA and is awaiting the Agency’s decision while preparing for a commercial launch later this year.
Geneoscopy appoints Don Hardison to its Board of Directors
ST. LOUIS, Feb. 28, 2023 /PRNewswire/ Geneoscopy, a developer of innovative diagnostics for gastrointestinal health, announced the appointment of Don Hardison to its board of directors. Mr. Hardison joins Geneoscopy’s Board of Directors with over 40 years of executive leadership experience in emerging and Fortune 500 diagnostics, biotechnology and life sciences companies.
Mr. Hardison served as president and CEO of Biotheranostics until its acquisition by Hologic in February 2021. Before that, he served as president, CEO, and director at Good Start Genetics. Mr. Hardison also held the position of president and CEO of Exact Sciences from 2000-2007, where he helped oversee the company’s initial public offering. His career includes multiple senior leadership positions at companies such as Labcorp.
“We are excited to welcome Don to our Board at this pivotal point in Geneoscopy’s evolution,” stated Andrew Barnell, Chief Executive Officer and Geneoscopy’s co-founder. “As an accomplished executive in our industry, Don’s expertise and insights will be valuable in guiding our efforts to bring innovative, noninvasive diagnostics for gastrointestinal health to the providers and patients who will benefit the most.”
For the past two years, Mr. Hardison has served as a strategic advisor to Geneoscopy. In his new role as a board member, Mr. Hardison will help guide Geneoscopy as it prepares for commercialization of its lead offering, a noninvasive, stool-based, at-home screening test to detect colorectal cancer and advanced adenomas in average-risk individuals. This past January, Geneoscopy submitted a Premarket Approval (PMA) application to the U.S. Food and Drug Administration for its test based on the favorable results from the pivotal CRC-PREVENT trial.
“As an advisor to Geneoscopy for the past two years, I have been impressed by its progress in executing its strategy. I am pleased to join Geneoscopy’s board at such an exciting time for the company, ” said Mr. Hardison. “The recent submission of the PMA – the Company’s first regulatory approval application – represents a significant milestone, and I look forward to supporting Geneoscopy’s commercial strategy as their momentum accelerates.”
About Geneoscopy Inc.
Geneoscopy Inc. is a life sciences company focused on developing diagnostic tests for gastrointestinal health. Leveraging its proprietary, patented stool-derived eukaryotic RNA (seRNA) biomarker platform, Geneoscopy’s mission is to empower patients and providers to transform gastrointestinal health through innovative diagnostics. Beyond colorectal cancer screening, Geneoscopy is developing tests for diagnosis, treatment selection, and therapy monitoring in other disease areas in partnership with leading universities and biopharmaceutical companies. For more information, visit www.geneoscopy.com and follow the company on LinkedIn.
Geneoscopy Inc. Forward-Looking Statements
This release includes information about Geneoscopy’s future plans concerning its noninvasive molecular test that can detect colorectal cancer and precancerous adenomas, which constitute forward-looking statements. These forward-looking statements are based on the Company’s reasonable estimates of future results or trends. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict, many of which are outside the Company’s control. Geneoscopy’s actual results and financial condition may differ materially from those in the forward-looking statements. Although the Company believes its business plans and objectives reflected in or suggested by these forward-looking statements are reasonable, such plans or objectives may not be achieved. The actual results may differ substantially from the projected result.
Geneoscopy Submits Premarket Approval Application to FDA for its Noninvasive Colorectal Cancer RNA Biomarker Screening Test
FDA’s Decision Forthcoming for Innovative Test with Agency’s Breakthrough Device Designation
ST LOUIS, Jan. 24, 2023 / — Geneoscopy, Inc., a life sciences company focused on the development of diagnostic tests for gastrointestinal health, announced today it submitted a Premarket Approval (PMA) application to the U.S. Food and Drug Administration (FDA) for its noninvasive, stool-based, at-home screening test to detect colorectal cancer (CRC) and advanced adenomas (AA) in average-risk individuals. The FDA designated the test as a Breakthrough Device in January 2020.
“Over 50 million Americans between the ages of 45 and 85 are eligible to be screened for CRC.1,2 Unfortunately, despite CRC being the second leading cause of cancer death in the U.S., millions of eligible Americans do not get screened – many due to a lack of knowledge of the importance of screening, lack of access to screening and concerns about the invasive nature of options like colonoscopy,” said Dr. Erica Barnell, Chief Science Officer and Geneoscopy’s co-founder. “We are committed to closing this gap for patients who require screening and look forward to working with the FDA to bring a convenient and reliable screening option to patients that may allow for earlier detection and treatment – and potentially save lives.”
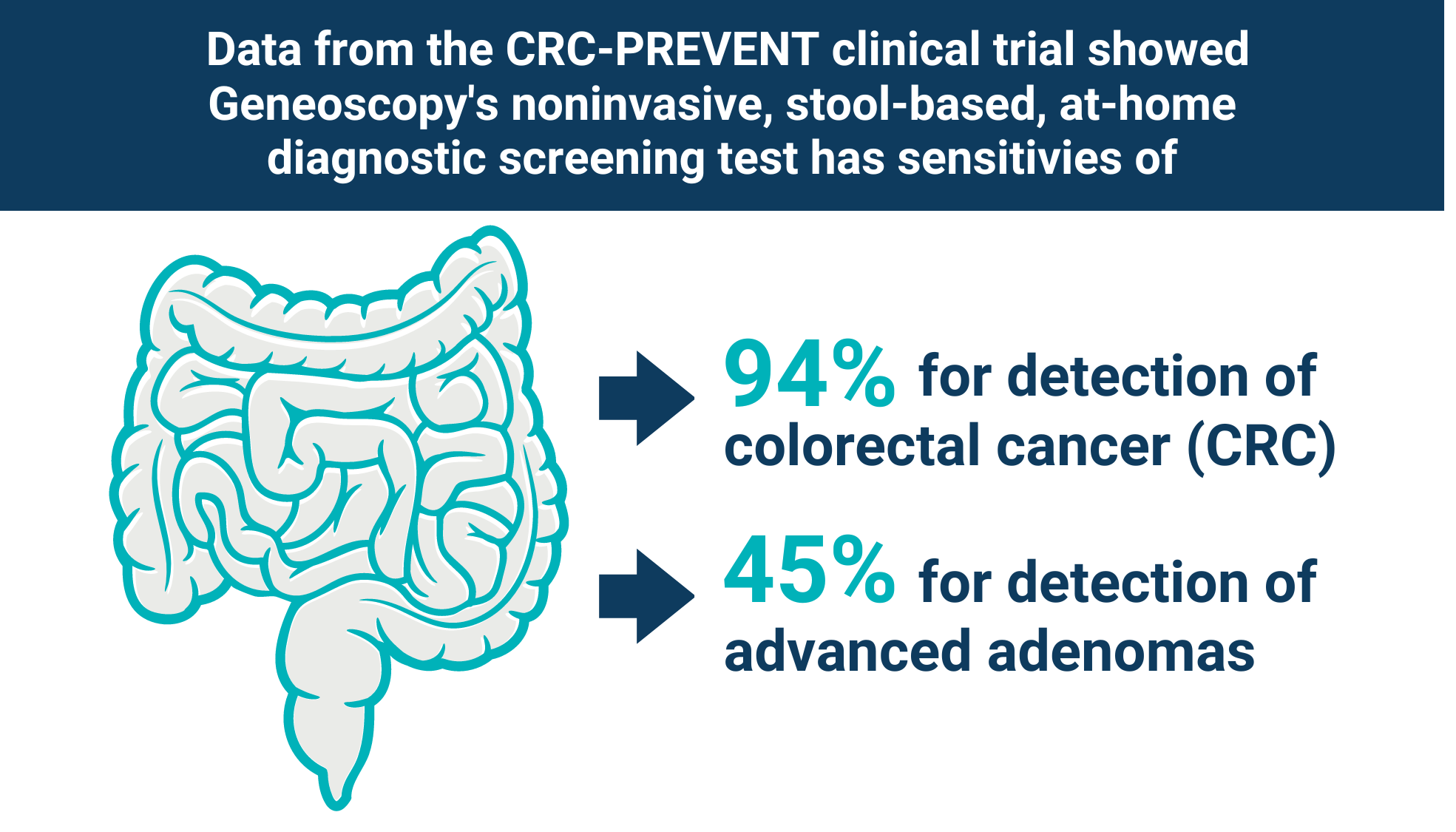
The PMA submission is based on the favorable results from the pivotal CRC-PREVENT trial, in which Geneoscopy’s test demonstrated 94% sensitivity for CRC and 45% sensitivity for advanced adenomas, representing the highest sensitivity profile reported to date for any noninvasive CRC screening test in a prospective clinical study.
Geneoscopy’s noninvasive stool-based at-home screening test demonstrated the highest sensitivity profile reported for any noninvasive CRC screening test in a prospective clinical study.
Additionally, in the 45-49 age population, Geneoscopy’s test demonstrated 100% sensitivity for cancer and 44% sensitivity for advanced adenomas, at an 89% specificity. CRC-PREVENT is the first prospective study of a stool-based test to report CRC sensitivity in patients ages 45-49, an age group most recently added to the recommended screening population by the United States Preventive Services Task Force and the American Cancer Society. The robust sensitivity profile for CRC and advanced adenomas will offer significant value to this patient population.
“The completion of our PMA submission is a major milestone – Geneoscopy’s first regulatory approval application,” said Andrew Barnell, Chief Executive Officer and Geneoscopy’s co-founder. “This product and the years of effort from our team that have brought us here exemplify the dedication to our mission to empower patients and providers to transform gastrointestinal health through innovative diagnostics. The impact this test can have on patient health is beyond exciting, as is the validation of our stool RNA platform. We believe this platform will yield additional tests, including increasingly more precise CRC screening and high-value diagnostic testing for other gastrointestinal indications.”
About CRC-PREVENT
CRC-PREVENT was a Phase 3 prospective, single-arm study designed to evaluate the efficacy of Geneoscopy’s noninvasive, stool-based, at-home screening test to detect colorectal cancer and advanced adenomas in average-risk individuals. Using a collection kit, participants submitted self-collected stool samples via express delivery and underwent an optical colonoscopy examination. All significant lesions discovered during the colonoscopy were biopsied or removed and sent for histopathology. A comparative analysis was conducted to determine sensitivities and specificities for colorectal cancer, advanced adenomas, non-advanced adenomas, hyperplastic polyps, and colonoscopies with no findings.
About Colorectal Cancer & Screening
Responsible for over 50,000 deaths annually, colorectal cancer (CRC) is the second leading cause of cancer death in the United States.CRC usually begins as a growth (or polyp) that may or may not develop into cancer over time. Early detection and treatment are crucial to improve survival; however, many newly diagnosed patients suffer from advanced disease. Colonoscopy remains the gold standard for CRC screening in the U.S. Yet this method is frequently met with patient aversion due to its required bowel preparation, sedation, and potential time away from work. Currently available noninvasive screening methods demonstrate lower sensitivity to detect early-stage CRC and high-risk precancerous lesions, including advanced adenomas, which are estimated to be a precursor in 95 percent of CRC cases.3
About Geneoscopy Inc.
Geneoscopy Inc. is a life sciences company focused on developing diagnostic tests for gastrointestinal health. Leveraging its proprietary, patented stool-derived eukaryotic RNA (seRNA) biomarker platform, Geneoscopy’s mission is to empower patients and providers to transform gastrointestinal health through innovative diagnostics. Beyond colorectal cancer screening, Geneoscopy is developing tests for diagnosis, treatment selection, and therapy monitoring in other disease areas in partnership with leading universities and biopharmaceutical companies. For more information, visit www.geneoscopy.com and follow the company on LinkedIn.
Geneoscopy Inc. Forward-Looking Statements
The information in this release includes information about Geneoscopy’s future plans concerning its noninvasive molecular test that can detect colorectal cancer and precancerous adenomas, which constitute forward-looking statements. These forward-looking statements are based on the Company’s reasonable estimates of future results or trends. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict, many of which are outside the Company’s control. Geneoscopy’s actual results and financial condition may differ materially from those indicated in the forward-looking statements. Although the Company believes that its business plans and objectives reflected in or suggested by these forward-looking statements are reasonable, such plans or objectives may not be achieved, and the actual results may differ substantially from the projected results.
References
- Colorectal Cancer Screening in U.S. Seniors ages 76-84 Years. (2016, August 1). NCBI. Retrieved January 19, 2023, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4491009/
- Estimating the Screening-Eligible Population Size, Ages 45-74, at Average Risk to Develop Colorectal Cancer in the United States. (n.d.). PubMed. Retrieved January 19, 2023, from https://pubmed.ncbi.nlm.nih.gov/32029430/
- Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jun. (Evidence Syntheses, No. 135.) 1, Introduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK373586/
Correction 01/31/23: Geneoscopy’s test received the FDA’s Breakthrough Device Designation in January 2020.
Geneoscopy’s Noninvasive Colorectal Cancer Screening Test Demonstrates High Sensitivity and Specificity in Large Pivotal Clinical Trial
Colorectal Cancer and Advanced Adenoma Sensitivity Results are the Highest Reported from any Prospective Pivotal Study for a Noninvasive Screening Test
Innovative RNA Biomarker Screening Test’s Premarket Approval Submission Planned for Early Q1 2023
ST. LOUIS, Jan. 10, 2023 /PRNewswire/ — Geneoscopy Inc., a life sciences company focused on the development of diagnostic tests for gastrointestinal health, today announced favorable results from the CRC-PREVENT trial – a pivotal clinical trial evaluating the efficacy of its noninvasive, stool-based, at-home diagnostic screening test to detect colorectal cancer (CRC) and advanced adenomas (AA) in average-risk individuals. In the trial, Geneoscopy’s stool-based screening test met the clinical endpoints across all primary outcome measures, including sensitivity and specificity for CRC and AA.
The CRC-PREVENT trial included 8,289 individuals with diverse racial, ethnic, and socioeconomic backgrounds across more than 2,900 zip codes in all lower 48 states, with colonoscopies performed in more than 3,800 endoscopy centers, which reflect the diversity of gastroenterology practices across America. Efficacy results from the study include:
- 94% sensitivity for detecting CRC
- 45% sensitivity for detecting AA
- 88% specificity for no findings on a colonoscopy
These sensitivity results are the highest reported for any noninvasive CRC screening test in any prospective registrational clinical study completed to date. Of note, CRC-PREVENT is the first prospective clinical study wherein a stool-based test demonstrated the ability to detect CRC amongst 45-49-year-olds in a US population.1 Additionally, CRC and AA sensitivity performance results for Geneoscopy’s test exceed those recently reported from a large clinical study for a blood-based test.2 Moreover, a recent study suggests, when given a choice, patients are more willing to comply with a stool-based screening test than a blood-based test. This was primarily attributed to the greater ability to complete a stool test at home.3
Importantly, given Geneoscopy’s decentralized clinical trial approach, the demographics of the patients who enrolled and completed the CRC-PREVENT study are more reflective of the socioeconomic and racial diversity of the country than most conventional, centralized trials. The diversity of the patient cohort confirms the test’s performance across different demographic groups and advances the important goal of increased access to healthcare innovation for historically underserved populations.
Geneoscopy’s test, performed in its St. Louis laboratory, uses a novel, proprietary method to stabilize and extract eukaryotic RNA biomarkers from stool samples that may allow for improved diagnosis and management of gastrointestinal diseases such as CRC. The FDA granted the test its Breakthrough Device Designation in January 2020.
“The use of our patented RNA biomarker technology is a first in CRC screening. The large-scale prospective clinical study data demonstrate that this noninvasive CRC screening test can accurately detect if people have cancer and if they have advanced adenomas that put them at higher risk of developing cancer. These results provide further evidence that our test may allow patients to get appropriate treatment, in some cases, even before cancer develops,” said Dr. Erica Barnell, Chief Science Officer and Geneoscopy’s co-founder. “Our sincerest gratitude goes to all who participated in or were involved with this trial. We look forward to submitting a Premarket Approval application to the FDA to make this cutting-edge innovation available to the millions of Americans eligible to be screened for CRC.”
Despite CRC being this country’s second leading cause of cancer death, millions of eligible Americans do not get screened – many due to a lack of access or avoidance of invasive options like colonoscopies.
“Colonoscopy screening rates declined during the pandemic, stressing the need for noninvasive screening options. That’s why noninvasive tests, allowing for collection to be done at home, have become a critical tool in the battle against CRC, as they make screening easier and more accessible,” noted Dr. David Lieberman, Professor of Medicine, Division of Gastroenterology and Hepatology, Oregon Health Sciences University School of Medicine, and past president of the American Gastroenterology Association.4 “Geneoscopy’s test and the positive clinical trial results are promising because patients need additional convenient options that will accurately detect colon cancer, as well as advanced adenomas, before patients have cancer. If we can identify patients with advanced adenomas and remove those lesions, many cancers can be prevented. I’m hoping to have a new and highly reliable test available for patients soon – one that will allow them to conveniently screen for CRC in their own homes.”
Geneoscopy’s test is not yet available for sale and is not yet approved by the U.S. Food and Drug Administration (FDA). A Premarket Approval submission to the FDA is planned for the first quarter of 2023.
About CRC-PREVENT
CRC-PREVENT was a Phase 3 prospective, single-arm study designed to evaluate the efficacy of Geneoscopy’s noninvasive, at-home diagnostic screening test to detect colorectal cancer and advanced adenomas in average-risk individuals aged 45 years and older. Using a collection kit, participants submitted self-collected stool samples via express delivery and underwent an optical colonoscopy examination. All significant lesions discovered during the colonoscopy were biopsied or removed and sent for histopathology. A comparative analysis was conducted to determine sensitivities and specificities for colorectal cancer, advanced adenomas, non-advanced adenomas, benign hyperplastic polyps, and colonoscopies with no findings.
About Colorectal Cancer & Screening
Responsible for over 50,000 deaths annually, colorectal cancer (CRC) is the second leading cause of cancer death in the United States. CRC usually begins as a growth (or polyp) that may or may not develop into cancer over time. Early detection and treatment are crucial to improve survival; however, many newly diagnosed patients suffer from advanced disease. Colonoscopy remains the gold standard for CRC screening in the U.S. Yet this method is frequently met with patient aversion due to its required bowel preparation, sedation, and potential time away from work. Currently available noninvasive screening methods demonstrate lower sensitivity to detect early-stage CRC and high-risk precancerous lesions, including advanced adenomas, which are estimated to be a precursor in 95 percent of CRC cases.5
About Geneoscopy, Inc.
Geneoscopy Inc. is a life sciences company focused on developing diagnostic tests for gastrointestinal health. Leveraging its proprietary, patented stool-derived eukaryotic RNA (seRNA) biomarker platform, Geneoscopy’s mission is to empower patients and providers to transform gastrointestinal health through innovative diagnostics. Beyond colorectal cancer screening, Geneoscopy is developing tests for diagnosis, treatment selection, and therapy monitoring in other disease areas in partnership with leading universities and biopharmaceutical companies. For more information, visit www.geneoscopy.com and follow the company on LinkedIn.
Geneoscopy Inc. Forward-Looking Statements
The information in this release includes information about Geneoscopy’s future plans concerning its noninvasive molecular test that can detect colorectal cancer and precancerous adenomas, which constitute forward-looking statements. These forward-looking statements are based on the Company’s reasonable estimates of future results or trends. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict, many of which are outside the Company’s control. Geneoscopy’s actual results and financial condition may differ materially from those indicated in the forward-looking statements. Although the Company believes that its business plans and objectives reflected in or suggested by these forward-looking statements are reasonable, such plans or objectives may not be achieved, and the actual results may differ substantially from the projected results.
Media Contact
Judy Pretto
Director, Marketing Communications
815.534.0521
media@geneoscopy.com
References
- Thomas F. Imperiale, John B. Kisiel, Steven H. Itzkowitz, Bradley Scheu, Emma Kate Duimstra, Sandra Statz, Barry M. Berger, Paul J. Limburg; Specificity of the Multi-Target Stool DNA Test for Colorectal Cancer Screening in Average-Risk 45–49 Year-Olds: A Cross-Sectional Study. Cancer Prev Res (Phila) 1 April 2021; 14 (4): 489–496. https://doi.org/10.1158/1940-6207.CAPR-20-0294
- Guardant. (2022, December 15). Guardant Health announces positive results from pivotal ECLIPSE study evaluating a blood test for the detection of colorectal cancer [Press release.] Retrieved from: https://investors.guardanthealth.com/press-releases/press-releases/2022/Guardant-Health-announces-positive-results-from-pivotal-ECLIPSE-study-evaluating-a-blood-test-for-the-detection-of-colorectal-cancer/default.aspx
- Young GP, Chen G, Wilson CJ, et al. “Rescue” of Nonparticipants in Colorectal Cancer Screening: A Randomized Controlled Trial of Three Noninvasive Test Options. Cancer Prev Res (Phila). 2021;14(8):803-810. doi:10.1158/1940-6207.CAPR-21-0080
- Dr. David Lieberman is a member of Geneoscopy’s Scientific Advisory Board.
- Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016 Jun. (Evidence Syntheses, No. 135.) 1, Introduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK373586/0
Correction 01/31/23: Geneoscopy's test received the FDA's Breakthrough Device Designation in January 2020.
Geneoscopy Receives Accreditation from the College of American Pathologists (CAP) for its St. Louis Clinical Laboratory
Adds CAP Accreditation to Clinical Laboratory Improvement Amendments (CLIA) certification

ST. LOUIS, Dec. 14, 2022 / — Geneoscopy, Inc., a life sciences company focused on developing diagnostic tests for gastrointestinal health, today announced that the Accreditation Committee of the College of American Pathologists (CAP) has accredited the Company's clinical laboratory facility at its headquarters in St. Louis. This achievement follows a recent on-site inspection as part of the CAP Laboratory Accreditation Program.
"This is a major milestone reflective of Geneoscopy's commitment to laboratory excellence, safety, and quality, and driven by our dedicated team," said Eric Duncavage, M.D., Medical Director for Geneoscopy. "Geneoscopy is now CAP-accredited, as well as Clinical Laboratory Improvement Amendments (CLIA) certified, demonstrating that our laboratory processes meet the rigorous standards for clinical testing and ensure that patients receive the highest quality results."
The CAP Laboratory Accreditation Program was started in the early 1960s and is recognized by the U.S. federal government as being equal to or more stringent than its inspection program. During CAP accreditation, inspectors examine the laboratory's records and quality control procedures for the preceding two years. CAP inspectors also examine laboratory staff qualifications, equipment, facilities, safety program, and associated documents. CAP accreditation is awarded to facilities that meet the highest quality standards.
Once achieved, on-site inspections occur every two years to assess ongoing compliance with the CAP accreditation program requirements. The CAP Laboratory Accreditation Program is the gold standard for laboratory excellence and fosters an environment for continuous improvement and patient safety.
Geneoscopy earned Clinical Laboratory Improvement Amendments (CLIA) Certification earlier this year. CLIA certification confirms that Geneoscopy's clinical laboratory meets the federal regulations for clinical diagnostic testing, ensuring high quality and safety for patient testing. As part of the certification process, Geneoscopy completed analytical and clinical validation evaluating the accuracy and reliability of its patented colorectal cancer diagnostics platform.
"Delivering accuracy and consistency is imperative to us to ensure our healthcare providers and patients can rely on our testing capabilities," said Erica Barnell, Ph.D., Chief Science Officer and co-founder of Geneoscopy. "These clinical accreditations represent exceptional standards in care and laboratory excellence, and that is why we're so proud of our team that performs at these levels."
About Colorectal Cancer & Screening
Responsible for over 52,000 deaths annually, colorectal cancer (CRC) is the second leading cause of cancer death in the United States, according to the American Cancer Society. CRC usually begins as a growth (or polyp) that develops into cancer over time. Thus, early detection and treatment are crucial to improving survival. Colonoscopy remains the gold standard for CRC screening in the U.S. Yet this method is frequently met with patient aversion due to its required bowel preparation, sedation, and potential time away from work, resulting in low patient compliance. Current noninvasive screening methods demonstrate lower sensitivity to detect early-stage CRC and high-risk precancerous lesions, including advanced adenomas, which are estimated to be a precursor in 95 percent of CRC cases.[1]
About Geneoscopy Inc.
Geneoscopy Inc. is a life sciences company focused on developing diagnostic tests for gastrointestinal health. Geneoscopy's lead assay uses stool-derived eukaryotic RNA (seRNA) to detect colorectal cancer and precancerous adenomas. The U.S. FDA awarded this device its Breakthrough Device Designation for its ability to reduce morbidity associated with colorectal cancer through advanced adenoma detection. Indicative of its breakthrough status, initial trials suggest that the test can detect these precancerous lesions at a higher rate than that demonstrated by all existing noninvasive screening tests in their respective studies. Visit geneoscopy.com to learn more.
Geneoscopy Inc. Forward-Looking Statements
This release includes information about Geneoscopy's plans concerning its noninvasive molecular test that can detect colorectal cancer and precancerous adenomas and constitute forward-looking statements. These forward-looking statements are based on the Company's reasonable estimates of future results or trends. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict, many of which are outside the Company's control. Geneoscopy's actual results and financial condition may differ materially from those indicated in the forward-looking statements. Although the Company believes that its business plans and objectives reflected in or suggested by these forward-looking statements are reasonable, such plans or objectives may not be achieved, and the actual results may differ substantially from the projected results.
About the College of American Pathologists
As the world's largest organization of board-certified pathologists and a leading provider of laboratory accreditation and proficiency testing programs, the College of American Pathologists (CAP) serves patients, pathologists, and the public by fostering and advocating excellence in the practice of pathology and laboratory medicine worldwide. For more information, read the CAP Annual Report at cap.org.
Clinical Laboratory Improvement Amendments (CLIA) Certification
The Clinical Laboratory Improvement Amendments (CLIA) regulate laboratory testing and require clinical laboratories to be certified by the Center for Medicare and Medicaid Services (CMS) before they can accept human samples for diagnostic testing. Under the U.S. Department of Health and Human Services Centers for Medicare and Medicaid Services provisions, the Missouri Department of Public Health granted Geneoscopy initial registration for high complexity testing through the CLIA Certification Program and follows Missouri State laboratory licensure.
[1] Lin JS, Piper MA, Perdue LA, et al. Screening for Colorectal Cancer: A Systematic Review for the U.S. Preventive Services Task Force [Internet]. Rockville (M.D.): Agency for Healthcare Research and Quality (U.S.); 2016 Jun. (Evidence Syntheses, No. 135.) 1, Introduction. Available from: https://www.ncbi.nlm.nih.gov/books/NBK373586/A
